Introduction
Disorders of the Achilles tendon are common in active people, competitive and recreational athletes alike, but they can occur in less active people. As the largest tendon in the body, the Achilles experiences repetitive strain from running, jumping, and sudden acceleration or deceleration, so is susceptible to rupture and degenerative changes. This article aims to describe the anatomy and diagnostic evaluation of the Achilles tendon, and to discuss the best available evidence to help in the management of Achilles tendon disorders.
What are Achilles Tendon Disorders?
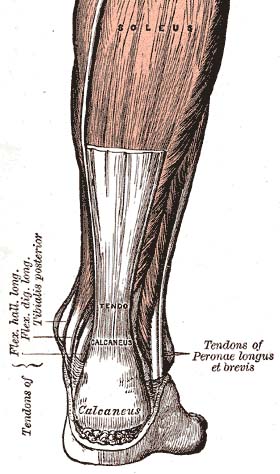 The Achilles tendon is the strongest tendon in the body (Doral et al., 2010) serving both the gastrocnemius and soleus muscles. It begins near the mid-calf and inserts posteriorly at the calcaneus (heel bone). In the region where the tendon joins the bone, there is an amalgam called the enthesis organ, in which the tissue is a composite of bone and tendon (Benjamin et al., 2004). Kager’s fat pad is located anterior to the Achilles tendon and posterior to the calcaneus, forms the superior border of this enthesis organ, and protects the blood vessels of the Achilles tendon (Benjamin et al., 2004). The fat pad may also provide a mechanical advantage by increasing the lever angle of the Achilles tendon during plantar flexion (Andres et al., 1985).
The Achilles tendon is the strongest tendon in the body (Doral et al., 2010) serving both the gastrocnemius and soleus muscles. It begins near the mid-calf and inserts posteriorly at the calcaneus (heel bone). In the region where the tendon joins the bone, there is an amalgam called the enthesis organ, in which the tissue is a composite of bone and tendon (Benjamin et al., 2004). Kager’s fat pad is located anterior to the Achilles tendon and posterior to the calcaneus, forms the superior border of this enthesis organ, and protects the blood vessels of the Achilles tendon (Benjamin et al., 2004). The fat pad may also provide a mechanical advantage by increasing the lever angle of the Achilles tendon during plantar flexion (Andres et al., 1985).
Unlike other tendons, the Achilles does not have a true synovial sheath but has a paratenon (a sheath of flexible connective tissue that allows for a gliding action). The paratenon and Achilles tendon are innervated by nerves from attached muscles and small fasciculi from cutaneous nerves; especially the sural nerve (Chen et al., 2009). The paratenon is a highly vascular structure, and along with the surrounding muscle complex supplies blood to the Achilles tendon (Ahmed et al., 1998).
Cadaveric studies suggest that there is an area 2-6 cm above the calcaneal insertion with a relatively poor blood supply and it is argued that this predisposes the region to chronic inflammation and rupture (Ahmed et al., 1998; Chen et al., 2009). However, in-vivo studies have failed to demonstrate this ‘watershed’ area. Direct measurement of forces reveal loading in the Achilles tendon to be as high as 9 kilonewtons (up to 12.5 times body weight) during running, which probably contributes to its high rate of injury (Komi, 1990).
Tendonitis, Tendinopathy and Tendinosis
Tendinitis is a common term used with Achilles disorders. However, chronic overuse tendon injuries are not caused by inflammation; instead, histology typically shows tissue degeneration and disorganisation (Maffulli et al., 1998). Tendinopathy or tendinosis are therefore more accurate terms, with tendinopathy the clinical term and tendinosis its pathological equivalent.
Recently, it has been proposed that tendon injury occurs along a continuum (Cook & Purdam, 2009). Initially, reactive tendinopathy is caused by overload and results in a non-inflammatory response that thickens the tendon, reduces stress, and increases stiffness in response to overload. If overload continues, this leads to tendon disrepair and highly disorganised tissue and, finally, degenerative tendinopathy, with even greater cellular disorder (Cook & Purdam, 2009).
It is unclear if this degeneration within the tendon is the source of pain because many asymptomatic tendons show degenerative changes (Kannus & Jozsa, 1991). However, painful tendons show an increase in sensory and sympathetic nerves from the highly innervated paratenon and fat pad (Forsgren et al., 2005), which may be the source of pain in symptomatic tendinopathy.
 Anatomy of the Achilles Tendon
Anatomy of the Achilles Tendon
The Achilles tendon is the biggest and thickest tendon and like all tendons, comprises of mainly fibrous tissues which does not stretch under strain and it joins the calf muscles to the calcaneal bone of the ankle. The role of the Achilles tendon is to bring about plantarflexion (downward pointing) of the ankle, push-off phase of walking and running, and also to aid in shock absorption on jumping. The blood supply to this tendon comes from the calf muscles (gastronemius and soleus) proximally and distally from the tendon-bone insertion. These sites are also the areas where there are the most number of nerve endings, thus explaining why these are the common sites of pain.
Causes of Injury
The strain that the Achilles tendon undergoes ranges from 3 times one’s body weight (in walking) to as high as 12 times (in jumping). Due to such high levels of strain, injuries tend to be multi-factorial. The table below outlines the causes of injury for Achilles tendinopathy.
|
Area |
Poor Training Technique |
Structural Abnormality |
Improper Footwear |
|
Cause(s) |
|
|
|
Nature of Pain
Achilles tendinopathy normally starts off as morning stiffness and pain on the first step either on the bony insertion or at the muscle-tendon junction. Soreness might also be felt on light pinching of the tendon. As the condition worsens, there might be pain on running and in severe cases, walking would also be painful. If this pain or soreness is ignored, it might lead to a partial or complete rupture of the Achilles tendon which would require immediate surgical intervention and a long rehabilitation process.
Who gets Achilles Tendon Disorders?
Achilles tendinopathy generally occurs in the mid-portion of the tendon or less commonly at its insertion in the calcaneus; this differentiation is important because the treatments differ. Tendon rupture can be complete or partial and the treatments for both of these will also be discussed.
Achilles tendinopathy is described by many as an overuse injury, but this does not really cover the idea of finding the cause of the overuse in an individual. A change in training volume, training pattern, footwear or training surface (e.g. changing from treadmill running to road running) would be one of the commonest precipitatory factors for symptom onset and if addressed early, can often return the ‘de-compensated’ individual back to exercise without further complicated treatment.
Tendinopathy
The most common causes of Achilles disorders are mid-portion tendinopathy (55-65%), followed by insertional tendinopathy (20-25%) (Maffulli & Almekinders, 2007). Achilles tendon disorders can affect anyone, but they most commonly affect active people, especially those who participate in running or jumping sports. In a cohort study with an 11 year follow-up, Achilles tendon overuse injuries occurred in 29% of runners compared with 4% of non-runners; the age adjusted odds ratio was 10.0 in runners compared with controls (Kujala et al., 2005).
Age, male sex, and obesity have been cited as risk factors for Achilles tendon disorders, but a recent study of athletes over 40 years of age found no influence of any of these factors (Longo et al., 2009; Kraemer et al., 2012).
A study of military recruits found that decreased plantar flexion strength and extremes of dorsiflexion (too much or too little) were associated with Achilles disorders (Mahieu et al., 2006). Abnormal subtalar joint motion has also been found to contribute to mid-portion tendinopathy (Ryan et al., 2009). A positive family history raises the risk of Achilles tendinopathy almost five times, suggesting a possible genetic link (Kraemer et al., 2012) Medical factors that may be associated with Achilles injury include hypertension, hyperlipidemia, and diabetes – presumably secondary to glycation or systemic inflammation (Reddy, 2004).
Tendon Rupture
It is now recognised that most tendinopathies are rarely associated with one single factor, and the degenerative process that precedes rupture likely results from a variety of different pathways and causative factors. Degenerative changes are likely over age 35 years and contribute to the increased susceptibility to tendon rupture (Kannus & Jozsa, 1991). Achilles tendon ruptures are most common in men in the fourth to fifth decade of life, perhaps because degenerative changes have started but activity levels are still high (Hess, 2010). The incidence of Achilles tendon rupture is 7 injuries per 100 000 in the general population and 12 injuries per 100,000 in competitive athletes (Hess, 2010).
Finally, a history of Achilles rupture places the individual at a higher risk of injury to the contralateral Achilles (Aroen et al., 2004). Running, jumping, or sudden explosive or eccentric activities are the usual mechanisms for rupture (Kongsgaard et al., 2005). Drugs may be associated with Achilles rupture in less active older adults. A population based cohort study found that fluoroquinolone antibiotics are associated with 12 episodes of rupture per 100,000 treatment episodes (Sode et al., 2007).
Another population based drug safety study found that use of fluoroquinolones increased risk for tendon disorders (odds ratio 1.7, 95% confidence interval 1.4 to 2.0) and Achilles rupture (4.1, 1.8 to 9.6), whereas concomitant use of fluoroquinolones and oral corticosteroids significantly increased the risk of Achilles rupture (43.2, 5.5 to 341.1) (Corrao et al., 2006).
How Are Achilles Tendon Disorders Evaluated?
A thorough history and physical examination are the first steps in the diagnosis of Achilles tendon disorders. The history should include pattern of symptoms – onset, duration, cessation, plus alleviating and exacerbating factors. Ascertain the level of training, previous injury, and previous treatments. Also, the presence of risk factors, such as previous injury, family history, medical history, and drug use should be determined.
Tendinopathy
Individuals with tendinopathy generally describe pain or stiffness in the Achilles 2-6 cm above the calcaneal insertion (Maffulli et al, 2004). Morning stiffness is common, and the pain is usually worse with activity, although it may continue into rest. Less commonly, individuals will describe similar symptoms with point tenderness over the insertion of the Achilles on the calcaneus.
Inspection of the individual’s gait may elicit the presence of overt gait abnormalities. With the individual in a prone position, palpate the distal lower leg to assess areas of tenderness. Tenderness in the body of the tendon or directly over the insertion, with or without crepitus, suggests tendinopathy. Swelling around the tendon or crepitus with active motion may indicate inflammation of the paratenon. Tendinopathy and paratendinopathy may coexist (Paavola & Jarvinnen, 2005). In isolated paratendinopathy, there is local thickening of the paratenon. Finally, assess range of motion (passive and active) and strength testing to plantar flexion, dorsiflexion, eversion, and inversion, along with subtalar mobility to evaluate for restrictions to motion or muscle weakness, which would predispose to re-injury. It is essential to compare the injured limb with the contralateral non-injured limb to appreciate subtle differences.
Severity of tendinopathy, as well as response to treatment, can be assessed by using a validated outcome measure such as the Victorian Institute of Sport assessment (VISA-A), which consists of eight items to assess stiffness, pain, and function (Robinson et al., 2001).
Rupture
Classically individuals with complete tendon rupture will describe the feeling of ‘being shot or hit in the back of the leg,’ typically while performing an explosive running or jumping manoeuvre, with immediate pain and an inability to continue their current activity.
Because gravity and activity of the tibialis posterior, peroneals, and long toe flexors can cause active plantar flexion, individuals should be examined for suspected rupture while they are prone. Ecchymosis suggests tendon rupture, and a palpable defect may exist within the first hours of rupture. Tendon rupture can be confirmed with the calf squeeze test, where the examiner gently squeezes the individual’s calf muscles with the palm of the hand – if the tendon is intact, plantar flexion will occur, if torn the ankle will remain still. Test both legs to assess for differences. Sensitivity and specificity of this test have been measured at 0.96 (0.91 to 0.99) and 0.93 (0.76 to 0.99), respectively (Thompson, 1962; Maffulli, 1998; AAOS, 2009).
What Is The Differential Diagnosis of Posterior Heel Pain?
Posterior heel pain can be a diagnostic challenge – consider Achilles tendinopathy (mid-portion or insertional) and tendon rupture (partial or complete). Achilles disorders will localise pain to the Achilles tendon, typically along its course from the insertion on the heel to its transition into the conjoined tendon of the gastrocnemius and soleus. However, differential diagnoses include retrocalcaneal bursitis or enthesitis (shows up as a prominent warm area of the upper back part of the heel), retro-Achilles bursitis (pain is near the skin surface and the area back of the heel is warm), paratendonitis, plantaris muscle injury, posterior ankle impingement, and sural nerve impingement or entrapment.
Retrocalcaneal bursitis may occur near the distal insertion of the Achilles and may mimic insertional tendinopathy, and both disorders can occur simultaneously. In Achilles enthesitis, in addition to the tendon findings, an effusion is often present in the retrocalcaneal bursa. This condition can be investigated using three finger palpation. The middle finger and thumb are placed on each side of the Achilles while the index finger palpates the distal tendon; fluctuation palpated with the index finger can indicate effusion of the retrocalcaneal bursa (Alvarez-Nenegyei & Canoso, 2004).
The plantaris muscle is a vestigial rope-like structure seen in 7-20% of the population (Simpson et al., 1991). It lies deep to the proximal lateral gastrocnemius muscle, travels obliquely, and inserts near the medial border of the Achilles tendon; in some cases it fuses with the Achilles (Delgado et al., 2002). Injury to the plantaris can mimic the symptoms seen with Achilles tendon disorders and can be diagnosed with magnetic resonance imaging (MRI) or ultrasound (Helms et al., 1995; Leekam et al., 1999).
Posterior impingement of the ankle refers to impingement of the posterior talus by the posterior aspect of the tibia when the ankle is in extreme plantar flexion. In this condition, pain occurs in the posterior ankle but increases with passive plantar flexion of the ankle, unlike in Achilles tendinopathy, in which the pain lessens. Finally, sural nerve entrapment or impingement may cause pain in the posterior distal leg and may mimic Achilles pathology.
What Is The Role of Imaging in Achilles Tendon Disorders?
Most Achilles disorders are diagnosed clinically. However, imaging may be useful when the diagnosis is unclear or when trying to differentiate between complete or partial tendon rupture. Ultrasound and MRI are useful when clinical examination does not yield a definitive diagnosis.
MRI is useful in the diagnosis of tendon disorders because it can detect abnormalities in the entire locomotor unit, including the tendon, calcaneus, Achilles insertion, retrocalcaneal bursa, peritendinous tissues, and musculotendinous junction. MRI findings also correlate with findings at surgery and may be useful for surgical planning (Karjalainen et al., 2000).
Ultrasound can provide a dynamic assessment of the tendon and can evaluate for tissue neovascularisation. Perhaps more importantly, it can be used to guide percutaneous procedures and is therefore becoming a popular imaging tool. In a prospective blinded comparison study of ultrasound and MRI for identification of Achilles tendinopathy, both had similar specificity, but MRI had better sensitivity (95% v 80%) (Khan et al., 2003).
However, a recent retrospective study comparing MRI with physical examination found that MRI was less sensitive in the diagnosis of Achilles tendon rupture and may be useful only for operative planning (Garras et al., 2012). In a prospective longitudinal cohort study, ultrasound was used to measure neovascularisation before and after eccentric exercises to help predict patient outcome, with a decrease in neovascularity corresponding to patient improvement (DeVos et al., 2007). It has been suggested that, in trained hands, ultrasound is better for focused examinations or for guiding intervention, whereas MRI is better for global assessment of the tendon or for operative planning (Garcia et al., 2010).
What Are The Treatment Options?
Conservative treatment is generally considered first for most Achilles tendon disorders. The aims of treatment are load reduction and pain management. Individuals with tendinopathy should be advised to reduce or discontinue the offending activity, weight bear as tolerated, use a heel lift to effectively shorten the Achilles and reduce load, and use acetaminophen (paracetamol) as needed for pain. Individuals with complete rupture should be referred to a surgeon for advice about treatment options.
Additional treatments for specific Achilles tendon disorders are outlined below. If conservative measures fail, referral to a sports medicine or orthopaedic specialist should be considered.
Mid-portion Achilles Tendinopathy
Eccentric calf exercises have the most evidence and best outcomes for the treatment of mid-portion Achilles tendinopathy. A meta-analysis of 11 randomised controlled trials (RCTs) found that eccentric exercises improved pain, patient function, and satisfaction compared with control treatments, such as concentric exercises, stretching, splinting, and ultrasound (Woodley et al., 2007). Another systematic review of 16 RCTs of non-operative treatments for mid-portion Achilles tendinopathy found that eccentric exercises had the most evidence of effectiveness (Magnussen et al., 2009). Furthermore, a five year follow-up study of an RCT found sustained long term improvement with eccentric exercises performed according to Alfredson’s heel drop programme (Alfredson et al., 1998; Van der Plas et al., 2012).
Incorrect adherence an eccentric programme in terms of technique and compliance is the most common reason for these exercises to fail. As such it is advised to refer, even for one session, to a sports physiotherapist who can demonstrate the exercises, give the individual a programme to follow and hopefully address the details of the precipitating factors, if this has not been done in the initial consultation.
Does the addition of other treatments to eccentric exercises improve results? Two RCTs found that the addition of low energy shock wave treatment to eccentric exercises is more effective than eccentric exercise alone (Rasmussen et al., 2008; Rompe et al., 2009). Finally, the addition of low level laser therapy to eccentric exercises accelerated clinical recovery (Stergioloas et al., 2008). Therefore both of these treatments may be additive to eccentric exercise and may be useful to individuals who do not respond to the initial eccentric exercises.
Topical glyceryl trinitrate has been used to treat tendinopathy, but is it effective for Achilles tendinopathy? A meta-analysis of seven studies showed that glyceryl trinitrate reduces pain during activities of daily living in chronic tendinopathy, with an odds ratio of 4.44 (2.34 to 8.40), and in acute and chronic phases combined, with an odds ratio of 4.86 (2.62 to 9.02) (Gambito et al., 2010).
For the specific treatment of Achilles tendinopathy, another systematic review found two RCTs of topical glyceryl trinitrate alone in the treatment of chronic (more than six weeks) Achilles tendinopathy. One trial found a benefit of glyceryl trinitrate in terms of pain on activity or at night and tendon tenderness, but the other trial found no difference from placebo at six months on pain at rest or with activity (Gambito et al., 2010). Finally, another RCT comparing physical therapy plus topical glyceryl trinitrate with therapy alone found that addition of glyceryl trinitrate was of questionable benefit (Kane et al., 2008).
Despite evidence at the cellular level of the role of nitric oxide in tendon healing, results of clinical studies in human Achilles tendons have been conflicting, so further validation is needed.
In their article on Achilles tendon disorders, the authors discuss the use of glyceryl trinitrate patches in the management of Achilles tendinopathy. It is important to highlight that in the UK glyceryl trinitrate patches are not licensed for this indication.
Further information is available in Drug and Therapeutic Bulleting, DTB 2012;50:93-96, Management of chronic Achilles tendinopathy.
High Volume Image Guided Injection
High Volume Image Guided injection (HVIGI) (Chan et al., 2008; Humphrey et al., 2010; Maffulli et al., 2012) is a novel treatment option which is currently gaining popularity in the UK and some suggest it will become the treatment of choice for mid-portion Achilles tendinopathy. This is a very simple procedure carried out under Ultrasound Scanner which has colour Doppler to visualise the site of neovascularisation. The success rate measured on VISA-A is comparable to many of the other modalities but is less invasive.
Insertional Tendinopathy
Much of the evidence is for the treatment of mid-portion tendinopathy, and it is unclear if it directly translates to treatment of insertional tendinopathy. A systematic review of 11 studies concluded that conservative treatment, including eccentric loading exercises and shock wave therapy, should be attempted before operative intervention (Kearney & Costa, 2010).
A small pilot study to evaluate the effect of painful eccentric loading exercises without dorsiflexion (different from eccentric loading exercises for mid-portion tendinopathy) found that two thirds of the patients who performed such exercises improved clinically. Interestingly, combined disease (such as tendon, bursa, or enthesitis) at the insertion does not exclude a satisfactory response to this training regimen (Jonsson et al., 2008). Finally, a larger RCT of individuals with chronic insertional tendinopathy found that low energy shock wave therapy may be superior to eccentric loading exercises in improving functional outcome scores (Rompe et al., 2008). This treatment may be useful in patients with a poor initial response to eccentric loading exercises.
However, it is important to remember that insertional tendinopathy may be inflammatory (enthesitis) and is a key clinical and pathological feature of the spondyloarthropathies which include ankylosing spondylitis, psoriatic arthritis and reactive arthritis – all conditions which can affect fit active individuals. Confusion can arise between non-inflammatory and inflammatory tendinopathy as injury, through running or otherwise, can trigger onset of peripheral features of the spondyloarthropathies such as enthesitis.
Clues to an underlying inflammatory spondyloarthropathy include associated features such as morning spinal stiffness, psoriasis, joint synovitis or uveitis. Preceding urethral discharge or diarrhoea may suggest a reactive arthritis. However occasionally chronic insertional tendinopathy may be the only clinical manifestation when radiology revealing broad-based fluffy calcaneal spurs and the presence of HLA B27 gene are helpful pointers to the presence of an underlying spondyloarthropathy. It is important to make the distinction between non-inflammatory insertional and inflammatory insertional tendinopathy as the management of the latter is very different with a clear role for systemic medications including biologics such as anti-tumour necrosis factor antagonists.
Achilles Rupture
Complete Rupture
Surgery is often recommended for complete Achilles rupture, but there is some controversy about its long term effectiveness. A meta-analysis of six RCTs comparing minimally invasive surgery with conventional approaches found no significant difference in outcomes, although minimally invasive surgery resulted in fewer infections and greater patient satisfaction (McMahon et al., 2011). In a Cochrane systematic review, open surgery was associated with a much lower rate of re-rupture than conservative treatment but had a higher rate of complications (Khan & Carey-Smith, 2010). If surgical repair is performed, current evidence supports a minimally invasive technique, and evidence supports early weight bearing to improve functional outcome scores (Suchak et al., 2008).
Although surgery is generally considered the gold standard, two RCTs comparing surgery to conservative management with immobilisation for complete tendon rupture showed no significant difference after one year in functional outcomes (Nillson-Helander et al., 2010; Keating & Will, 2011). Another RCT of operative versus non-operative (accelerated functional rehabilitation programme) treatment of Achilles tendon rupture found that all outcome measures, including rate of re-rupture, were similar in both groups (Willits et al., 2010). In this study, all patients wore a walking boot with a 2 cm heel lift two weeks after injury; early range of motion and weight bearing as tolerated started at four to six weeks and strength exercises at six to eight weeks.
Finally, an RCT of early motion plus surgery versus early motion without surgery suggested that controlled early motion is an important part of treatment for a ruptured Achilles tendon (Twaddle & Poon, 2007). Therefore, recent studies indicate that good outcomes may be achieved without surgery, especially with accelerated functional rehabilitation and early motion. Maintain a high level of suspicion for deep venous thrombosis, the incidence of which is high after complete rupture (Nillson-Helander et al., 2009).
Because outcomes can be similar for conservative or surgical management, it is useful to know which patients are most suitable for surgery. Non-surgical management is generally best for older less active patients or those with poor skin integrity or wound healing problems (Khan & Carey-Smith, 2010). Surgical management is recommended for young people, active high-level athletes, and those in whom non-surgical management has been unsuccessful.
Partial Rupture
Much of the research into the treatment of Achilles rupture has been performed on complete tendon rupture rather than partial rupture. It is difficult to differentiate partial tears from tendinopathy. Imaging may help, but MRI findings can overlap substantially (Haims et al., 2000). Ultrasound can differentiate full thickness tears from partial thickness ones or tendinosis of the Achilles tendon with 92% accuracy (Hartgerink et al., 2001; Khan et al., 2003) so should be used in this situation.
Tendon repair can be slow and incomplete, and partial tendon ruptures often respond poorly to conservative measures. Surgery has therefore been the recommended treatment (Magnussen & Dunn, 2009) even though it has a long recovery period and greater incidence of complications. Recently, despite the apparent lack of effectiveness of platelet-rich plasma for treating Achilles tendinopathy, two case reports found a successful return to sports and long term outcome after treatment of a partial Achilles rupture with the injection of platelet-rich plasma followed by a progressive rehabilitation programme (Filardo et al., 2010; Sampson et al., 2011). Platelet-rich plasma may be a useful addition to the current conservative management options for partial Achilles tendon ruptures, although more definitive studies are needed before it can be recommended.
What Is The Prognosis?
Most people who develop Achilles tendinopathy will improve with conservative treatment. In general, significant decreases in pain and improvement in function occur after 12 weeks of intervention (Roos et al., 2004). A long term follow-up study showed that 85% of patients with Achilles tendinopathy had full normal function and continued to be asymptomatic eight years after injury (Paavola et al., 2000).
Surgery for tendinopathy is reserved for individuals who do not respond after six months of conservative measures. Nevertheless, 24 of the 83 patients in the long term follow-up study did not respond to conservative treatment and underwent surgery (Paavola et al., 2000).
Treatment of complete Achilles rupture is controversial, but good outcomes have been seen after both operative and non-operative approaches (Nillson-Helander et al., 2010; Willits et al., 2010; Keating & Will, 2011). Regardless of treatment approach, functional deficits may persist for up to two years (Olsson et al., 2011). It is therefore important to advise patients of the potential for a long recovery.
The Role of Orthotics
There is currently no research to ascertain the effectiveness of orthotics with regards to Achilles tendinopathy, but anecdotal evidence suggests that some individuals may benefit from customised orthotics.
Prevention Is Better Than Cure
Achilles injury can be prevented through regular stretches, proper warm-up and proper biomechanics. Choosing the right kind of footwear that supports your arch and gives adequate cushioning can help reduce the strain and loading of the tendon. Staying in shape and proper progression of one’s training is the best way to prevent Achilles injury.
References
| Ahmed, I.M., Lagopoulos, M., McConnell, P., Soames, R.W. & Sefton, G.K. (1998) Blood Supply of the Achilles Tendon. Journal of Orthopaedic Research. 16, pp.591-596. |
| Alfredson, H., Pietila, T., Jonsson, P. & Lorentzon, R. (1998) Heavy-load Eccentric Calf Muscle Training for the Treatment of Chronic Achilles Tendinosis. American Journal of Sports Medicine. 26, pp.360-366. |
| Alvarez-Nenegyei, J. & Canoso, J.J. (2004) Heel Pain: Diagnosis and Treatment, Step by Step. Cleveland Clinical Journal of Medicine. 73, pp.465-471. |
| American Academy of Orthopaedic Surgeons. (2009) The Diagnosis and Treatment of Acute
Achilles Tendon Rupture: Guideline and Evidence Report. Available from World Wide Web: <http://www.aaos.org/research/guidelines/atrguideline.pdf.> [Accessed: 12 February, 2013]. |
| Andres, K.H., von During, M. & Schmidt, R.F. (1985) Sensory Innervation of the Achilles Tendon by Group III and IV Afferent Fibers. Anatomy & Embryology. 172, pp.145-146. |
| Årøen, A., Helgø, D., Granlund, O.G. & Bahr, R. (2004) Contralateral Tendon Rupture Risk is Increased in Individuals with a Previous Achilles Tendon Rupture. Scandinavian Journal of Medicine and Science in Sports. 14, pp.30-33. |
| Benjamin, M., Moriggl, B., Brenner, E., Emery, P., McGonagle, D. & Redman, S. (2004) The “Enthesis Organ” Concept. Arthritis & Rheumatology. 50, pp.3306-3313. |
| Canoso, J.J., Liu, N., Traill, M.R. & Runge, V.M. (1988) Physiology of the Retrocalcaneal Bursa. Annals of Rheumatological Disease. 47, pp.910-912. |
| Carcia, C.R., Martin, R.L., Houck, J. & Wukich, D.K. (2010) Achilles Pain, Stiffness, and Muscle Power Deficits: Achilles Tendinitis Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health from the Orthopaedic Section of the American Physical Therapy Association. Journal of Orthopaedic and Sports Physical Therapy. 40, pp.A1-A26. |
| Chan, O., O’Dowd, D., Padhiar, N., Morrissey, D., King, J., Jalan, R., Maffulli, N. & Crisp, T. (2008) High Volume Image Guided Injections in Chronic Achilles Tendinopathy. Disability and Rehabilitation. 30(20-22), pp.1697-1708. |
| Chen, T.M., Rozen, W.M., Pan, W.R., Ashton, M.W., Richardson, M.D. & Taylor, G.I. (2009) The Arterial Anatomy of the Achilles Tendon: Anatomical Study and Clinical Implications. Clinical Anatomy. 22, pp.377-385. |
| Cook, J.L. & Purdam, C.R. (2009) Is Tendon Pathology a Continuum? A Pathology Model to Explain the Clinical Presentation of Load Induced Tendinopathy. British Journal of Sports Medicine. 43, pp.409-416. |
| Corrao, G., Zambon, A., Bertu, L., Mauri, A., Paleari, V., Rossi, C., et al. (2006) Evidence of Tendinitis Provoked by Fluoroquinolone Treatment: A Case-control Study. Drug Safety. 29, pp.889-896. |
| DeVos, R.J., Weir, A., Cobben, L.P. & Tol, J.L. (2007) The Value of Power Doppler Ultrasonography in Achilles Tendinopathy: A Prospective Study. American Journal of Sports Medicine. 35, pp.1696-1701. |
| Doral, M.N., Alam, M., Bozkurt, M., Turhan, E., Atay, O.A., Dönmez G, et al. (2010) Functional Anatomy of the Achilles Tendon. Knee Surg Sports Traumatol Arthrosc. 18, pp.638-643. |
| Filardo, G., Presti, M.L., Kon, E. & Marcacci, M. (2010) Nonoperative Biological Treatment Approach for Partial Achilles Tendon Lesion. Orthopedics. 33, pp.120-123. |
| Forsgren, S., Danielson, P. & Alfredson, H. (2005) Vascular NK-1 Receptor Occurrence in Normal and Chronic Painful Achilles and Patellar Tendons: Studies on Chemically Unfixed as well as Fixed Specimens. Regulatory Peptides 2005;126:173-81. |
| Gambito, E.D., Gonzalez-Suarez, C.B., Oquiñena, T.I. & Agbayani, R.B. (2010) Evidence on the Effectiveness of Topical Nitroglycerin in the Treatment of Tendinopathies: A SystematicReview. Archives of Physical Medicine and Rehabilitation. 91, pp.1291-1305. |
| Garras, D.N., Raikin, S.M., Bhat, S.B., Taweel, N. & Karanjia, H. (2012) MRI is Unnecessary for Diagnosing Acute Achilles Tendon Ruptures: Clinical Diagnostic Criteria. Clinical Orthopaedics and Related Research. 470, pp.2268-2273. |
| Haims, A.H., Schweitzer, M.E., Patel, R.S., Hecht, P. & Wapner, K.L. (2000) MR Imaging of the Achilles Tendon: Overlap of Findings in Symptomatic and Asymptomatic Individuals. Skeletal Radiology. 29, pp.640-645. |
| Hartgerink, P., Fessell, D.P., Jacobson, J.A. & van Holsbeeck, M.T. (2001) Full-versus Partial-thickness Achilles Tendon Tears: Sonographic Accuracy and Characterization in 26 Cases with Surgical Correlation. Radiology. 220, pp.406. |
| Helms, C.A., Fritz, R.C. & Garvin, G.J. (1995) Plantaris Muscle Injury: Evaluation with MR Imaging. Radiology. 195, pp.201-203. |
| Hess, G.W. (2010) Achilles Tendon Rupture: A Review of Aetiology, Population, Anatomy, Risk Factors, and Injury Prevention. Foot and Ankle Specialist. 3, pp.29-32. |
| Humphrey, J., Chan, O., Crisp, T., Padhiar, N., Morrissey, D., Twycross-Lewis, R., King, J. & Maffulli, N. (2010) The Short-term Effects of High Volume Image Guided Injections in Resistant Non-insertional Achilles Tendinopathy. Journal of Science and Medicine in Sport. 13(3), pp.295-298. |
| Jonsson, P., Alfredson, H., Sunding, K., Fahlstrom, M. & Cook, J. (2008) New Regimen for Eccentric Calf-muscle Training in Patients with Chronic Insertional Achilles Tendinopathy: Results of A Pilot Study. British Journal of Sports Medicine. 42, pp.746-749. |
| Kane, T.P., Ismail, M. & Calder, J.D. (2008) Topical Glyceryl Trinitrate and Noninsertional Achilles Tendinopathy: A Clinical and Cellular Investigation. American Journal of Sports Medicine. 36, pp.1160-1163. |
| Kannus, P. & Jozsa, L. (1991) Histopathological Changes Preceding Spontaneous Rupture of a Tendon. Journal of Bone and Joint Surgeons of America. 73, pp.1507-1525. |
| Karjalainen, P.T., Soila, K., Aronen, H.J., Pihlajamaki, H.K., Tynninen, O., Paavonen, T., et al. (2000) MR Imaging of Overuse Injuries of the Achilles Tendon. American Journal of Roentgenology. 175, pp.251-260. |
| Kearney, R. & Costa, M.L. (2010) Insertional Achilles Tendinopathy Management: A Systematic Review. Foot and Ankle International. 31, pp.689-694. |
| Keating, J.F. & Will, E.M. (2011) Operative versus Non-operative Treatment of Acute Rupture of Tendo Achillis: A Prospective Randomized Evaluation of Functional Outcome. Journal of Bone and Joint Surgery (British Volume). 93, pp.1071-1078. |
| Khan, K.M., Forster, B.B., Robinson, J., Cheong, Y., Louis, L., Maclean, L., et al. (2003) Are Ultrasound and Magnetic Resonance Imaging of Value in Assessment of Achilles Tendon Disorders? A Two Year Prospective Study. British Journal of Sports Medicine. 37, pp.149-153. |
| Khan, R.J. & Carey-Smith, R.L. (2010) Surgical Interventions for Treating Acute Achilles Tendon Ruptures. Cochrane Database Systematic Review. 9:CD003674. |
| Komi, P.V. (1990) Relevance of In-vivo Force Measurements to Human Biomechanics. Journal of Biomechanics 23(suppl 1), pp.23-34. |
| Kongsgaard, M., Aagaard, M., Kjaer, M. & Magnusson, S.P. (2005) Structural Achilles Tendon Properties in Athletes Subjected to Different Exercise Modes and in Achilles Tendon Rupture Patients. Journal of Applied Physiology. 99, pp.1965-1971. |
| Kraemer, R., Wuerfel, W., Lorenzen, J., Busche, M., Vogt, P.M. & Knobloch, K. (2012) Analysis of Hereditary and Medical Risk Factors in Achilles Tendinopathy and Achilles Tendon Ruptures: A Matched Pair Analysis. Archives of Orthopaedic Trauma and Surgery. 132, pp.847-853. |
| Kujala, U.M., Sarna, S. & Kaprio, J. (2005) Cumulative Incidence of Achilles Tendon Rupture and Tendinopathy in Former Elite Athletes. Clinical Journal of Sport and Medicine. 15, pp.133-135. |
| Leekam, R.N., Agur, A.M. & McKee, N.H. (1999) Using Sonography to Diagnose Injury of Plantaris Muscles and Tendons. American Journal of Roentgenology. 172, pp.185-189. |
| Longo, U.G., Rittweger, J., Garau, G., Radonic, B., Gutwasser, C., Gilliver, S.F., et al. (2009) No Influence of Age, Gender, Weight, Height, and Impact Profile in Achilles Tendinopathy in Masters Track and Field Athletes. American Journal of Sports Medicine. 37, pp.1400-1405. |
| Maffulii, N. & Almekinders, L.C. (2007) The Achilles Tendon. Springer. 43. |
| Maffulli, N. (1998) The Clinical Diagnosis of Subcutaneous Tear of the Achilles Tendon: A Prospective Study in 174 Patients. American Journal of Sports Medicine. 26, pp.266-270. |
| Maffulli, N., Khan, K.M. & Puddu, G. (1998) Overuse Tendon Conditions: Time to Change a Confusing Terminology. Arthroscopy. 14, pp.840-843. |
| Maffulli, N., Sharma, P. & Luscombe, K.L. (2004) Achilles Tendinopathy: Aetiology and Management. Journal of the Royal Society of Medicine. 97, pp.472. |
| Maffulli, N., Spiezia, F., Longo, U.G., Denaro, V. & Maffulli, G.D. (2012) High Volume Image Guided Injections for the Management of Chronic Tendinopathy of the Main Body of the Achilles Tendon. Physical Therapy in Sport. Nov 3. pii: S1466-853X(12)00078-8. |
| Magnussen, R.A., Dunn, W.R. & Thomson, A.B. (2009) Nonoperative Treatment of Midportion Achilles Tendinopathy: A Systematic Review. Clinical Journal of Sport Medicine. 19, pp.54-64. |
| Mahieu, N.N., Witvrouw, E., Stephens, V., Van Tiggelen, D. & Roget, P. (2006) Intrinsic Risk Factors for the Development of Achilles Tendon Overuse Injury: A Prospective Study. American Journal of Sports Medicine. 34, pp.226-235. |
| McMahon, S.E., Smith, T.O. & Hing, C.B. (2011) A Meta-analysis of Randomized Controlled Trials Comparing Conventional to Minimally Invasive Approaches for Repair of an Achilles Tendon Rupture. Journal of Foot and Ankle Surgery. 17, pp.211-217. |
| Nillson-Helander, H., Silbernagel, K.G., Thomee, R., Faxen, E., Olsson, N., Eriksson, B.I., et al. (2010) Acute Achilles Tendon Rupture: A Randomized Controlled Study Comparing Surgical and Nonsurgical Treatments using Validated Outcome Measures. American Journal of Sports Medicine. 38, pp.2186-2193. |
| Nillson-Helander, K., Thurin, A., Karlsson, J. & Eriksson, B.I. (2009) High Incidence of Deep Venous Thrombosis after Achilles Tendon Rupture: A Prospective Study. Knee Surgery, Sports Traumatology, Arthroscopy. 17, pp.1234-1238. |
| Olsson, N., Nillson-Helander, K., Karlsson, J., Eriksson, B.I., Thomee, R., Faxen, E., et al. (2011) Major Functional Deficits Persist 2 Years After Acute Achilles Tendon Rupture. Knee Surgery, Sports Traumatology, Arthroscopy. 19, pp.1385-1393. |
| Paavola, M. & Jarvinnen, T.A.H. (2005) Paratendinopathy. Foot Ankle Clinic of North America. 10, pp.279-292. |
| Paavola, M., Kannus, P., Paakkala, T., Pasanen, M. & Jarvinen, M. (2000) Long-term Prognosis of Patients with Achilles Tendinopathy. An Observational 8-year Follow-up Study. American Journal of Sports Medicine. 28, pp.634-642. |
| Rasmussen, S., Christensen, M., Mathiesen, I. & Simonson, O. (2008) Shockwave Therapy for Chronic Achilles Tendinopathy: A Double-blind, RCT of Efficacy. Acta Orthopaedica Scandinavica. 79, pp.249-256. |
| Reddy, G.K. (2004) Cross-linking in Collagen by Nonenzymatic Glycation Increases the Matrix Stiffness in Rabbit Achilles Tendon. Experimental Diabesity Research. 5, pp.143-153. |
| Robinson, J.M., Cook, J.L., Purdam, C., Visentini, P.J., Ross, J., Maffulli, N., et al. (2001) The VISA-A Questionnaire: A Valid and Reliable Index of the Clinical Severity of Achilles Tendinopathy. British Journal of Sports Medicine. 35, pp.335-341. |
| Rompe, J.D., Furia, J. & Maffulli, N. (2008) Eccentric Loading Compared with Shock Wave Treatment for Chronic Insertional Achilles Tendinopathy. A Randomized, Controlled Trial. Journal of Bone and Joint Surgery (American Volume). 90, pp.52-61. |
| Rompe, J.D., Furia, J. & Maffulli, N. (2009) Eccentric Loading versus Eccentric Loading Plus Shock-wave Treatment for Midportion Achilles Tendinopathy: A Randomized Controlled Trial. American Journal of Sports Medicine. 37, pp.463-470. |
| Roos, E.M., Engstrom, M., Lagerquist, A. & Soderberg, B. (2004) Clinical Improvement after 6 Weeks of Eccentric Exercise in Patients with Mid-portion Achilles Tendinopathy – A Randomized Trial with 1-year Follow-up. Scandinavian Journal of Medicine and Science in Sports. 14, pp.286-295. |
| Ryan, M., Grau, S., Krauss, I., Maiwald, C., Taunton, J. & Hortsmann, T. (2009) Kinematic Analysis of Runners with Achilles Mid-portion Tendinopathy. Foot and Ankle International 30, pp.1190-1195. |
| Sampson, S., Aufiero, D., Meng, M., Bledin, A., Gillette, T. & Zall, M. (2011) Platelet-rich Plasma Therapy as a First-line Treatment for Severe Achilles Tendon Tear: A Case Report. International Journal of Therapy and Rehabilitation. 18, pp.101-106. |
| Simpson, S.L., Hertzog, M.S. & Barja, R.H. (1991) The Plantaris Tendon Graft: An Ultrasound Study. Journal of Hand Surgery (American Volume). 16, pp.708-711.Delgado, G.J., Chung, C.B., Lektrakul, N., Azocar, P., Botte, M.J., Coria, D., et al. (2002) Tennis Leg: Clinical US Study of 141 Patients and Anatomic Investigation of Four Cadavers with MR Imaging and US. Radiology 224, pp.112-119. |
| Sode, J., Obel, N., Hallas, J. & Lassen, A. (2007) Use of Fluoroquinolones and Risk of Achilles Rupture: A Population Based Cohort Study. European Journal of Clinical Pharmacology. 63, pp.499. |
| Stergioloas, A., Stergiola, M., Aarskog, R., Lopes-Martin, R.A. & Bjordal, J.M. (2008) Effects of Low-level Laser Therapy and Eccentric Exercises in the Treatment of Recreational Athletes with Chronic Achilles Tendinopathy. American Journal of Sports Medicine. 36, pp.881-887. |
| Suchak, A.A., Bostick, G.P., Beaupre, L.A., Durand, D.C. & Jomha, N.M. (2008) The Influence of Early Weight-bearing Compared with Non-weight-bearing After Surgical Repair of the Achilles Tendon. Journal of Bone and Joint Surgery (American Volume). 90, pp.1876-1883. |
| Thompson, T.C. (1962) A Test for Rupture of the Tendo Achillis. Acta Orthopaedica Scandinavica. 32, pp.461-465. |
| Twaddle, B.C. & Poon, P. (2007) Early Motion for Achilles Tendon Ruptures: Is Surgery Important? A Randomized, Prospective Study. American Journal of Sports Medicine. 35, pp.2033-2038. |
| Van der Plas, A., de Jonge, S., de Vos, R.J., van der Heide, H.G., Verhaar, J.A., Weir, A., et al. (2012) A 5-year Follow-up Study of Alfredson’s Heel Drop Exercise Programme in Chronic Midportion Achilles Tendinopathy. British Journal of Sports Medicine. 46, pp.214-218. |
| Willits, K., Amendola, A., Bryant, D., Mohtadi, N.G., Giffin, J., Fowler, R., et al. (2010) Operative versus Nonoperative Treatment of Acute Achilles Tendon Rupture: A Multicenter Randomized Trial using Accelerated Functional Rehabilitation. Journal of Bone and Joint Surgery (American Volume). 92, pp.2767-2775. |
| Woodley, B.L., Newsham-West, R.J. & Baxter, G.D. (2007) Chronic Tendinopathy: Effectiveness of Eccentric Exercise. British Journal of Sports Medicine. 41, pp.188-198. |


